Our technologies
Advanced polymer platforms for medical devices


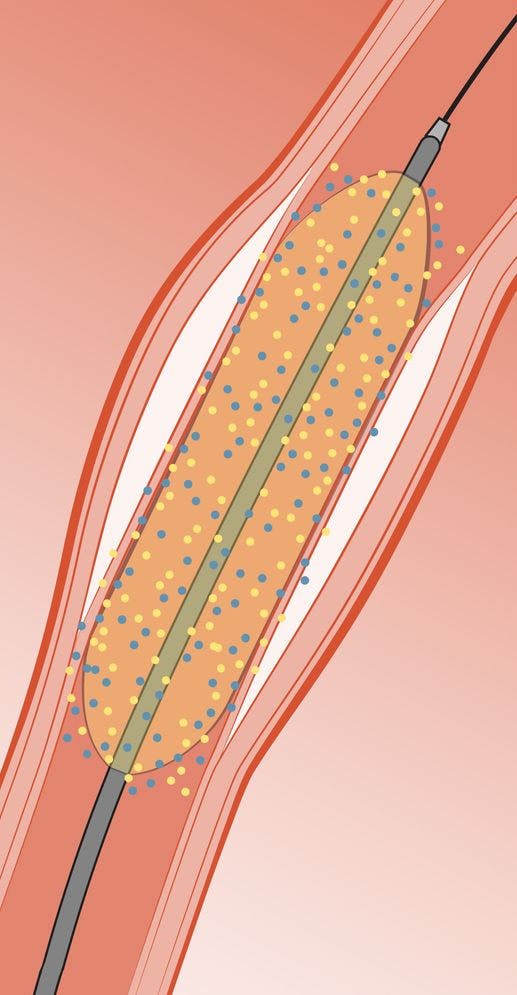
Arterius’ polymer coating provides the foundation for a significant improvement on DCBs

Delivery of most of the drug contained on the surface of the balloon to the tissue – some currently available DCBs deliver only a small fraction of the drug to the tissue, with most lost systemically during transit to the lesion or remaining on the surface of the balloon when it is withdrawn.

Delivery of more than one drug to the vessel wall – dual drug therapy provides the opportunity for long-term inhibition of restenosis and reduced risk of local thrombus formation.


Approved by regulators
Arterius’ DCB under development is using a balloon catheter that is already approved by regulators, and the coating technology may be applied to any PTA or PTCA balloon platform
Easily transferrable
Arterius’ polymer coating technology is compatible with both types of PTA balloon substrate on the market today (nylon and Pebax™). The platform will be easily transferrable to another balloon substrate, as required
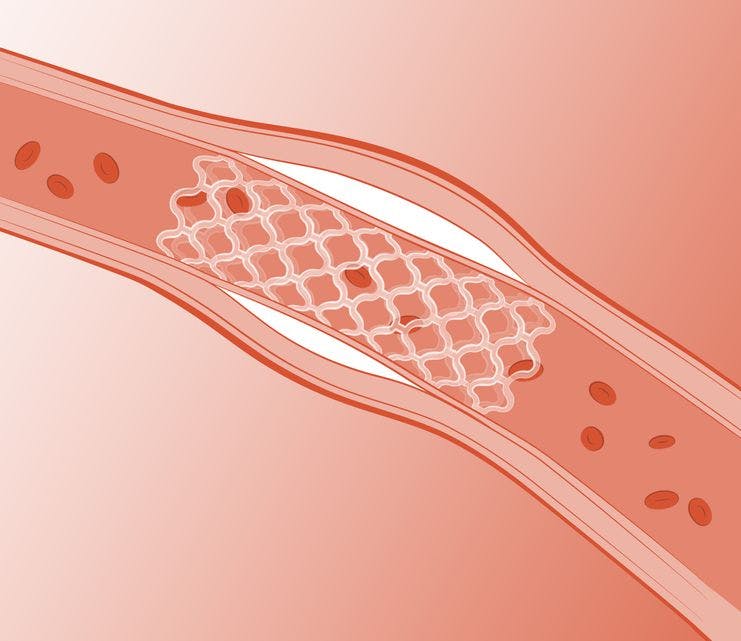
Arterius’ bioresorbable scaffold addresses the problems associated with metal stents and first-generation bioresorbable scaffolds


No long-term stent in the vessel to complicate future revascularisation procedures that may be required
No long-term occlusion of side branches of the stented vessel
ArterioSorb™ is made with PLLA, a universally-approved biocompatible polymer that is widely used for dissolving sutures and soft-tissue implants
Arterius’ patented ‘solid phase orientation’ production process enables ArterioSorb™ to address the issues associated with the early generation of bioresorbable scaffolds
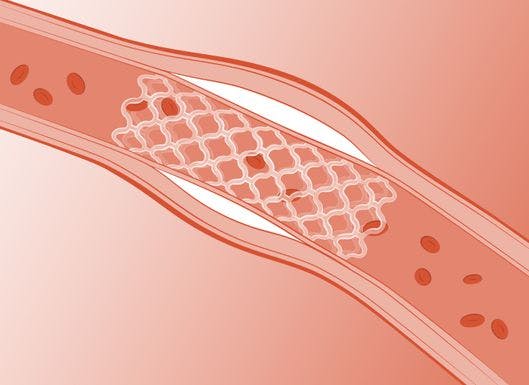
It provides comparable radial force to a metal stent for 3–6 months while the vessel remodels

Strut thickness of only 95 microns reduces risk of thrombosis – which can be further reduced by the application of anti-platelet drug using Arterius’ polymer coating
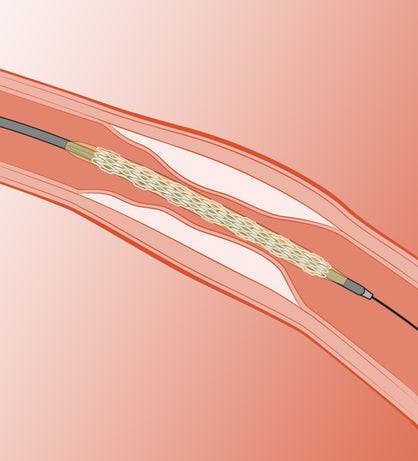
It is highly flexible, facilitating navigation to the target lesion through tortuous anatomy
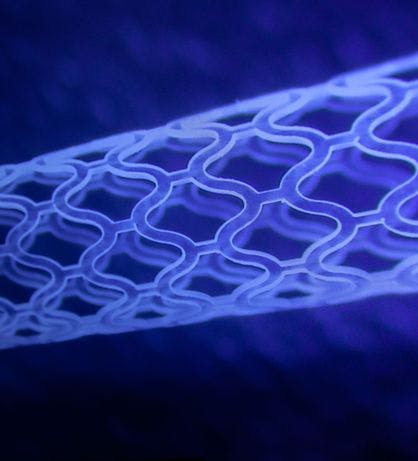
The scaffold design uses larger cells towards the extremities providing maximal flexibility and smaller cells in the centre to provide maximal structural support to the target lesion
The scaffold can be manufactured in a wide range of lengths with diameter from 2–8mm making it suitable for a broad range of lesions
![[object Object]](/_next/image?url=https%3A%2F%2Farterius-assets.s3.eu-west-1.amazonaws.com%2Farterius-assets%2F014717-B-141-676x158.jpg&w=1920&q=75)